ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição
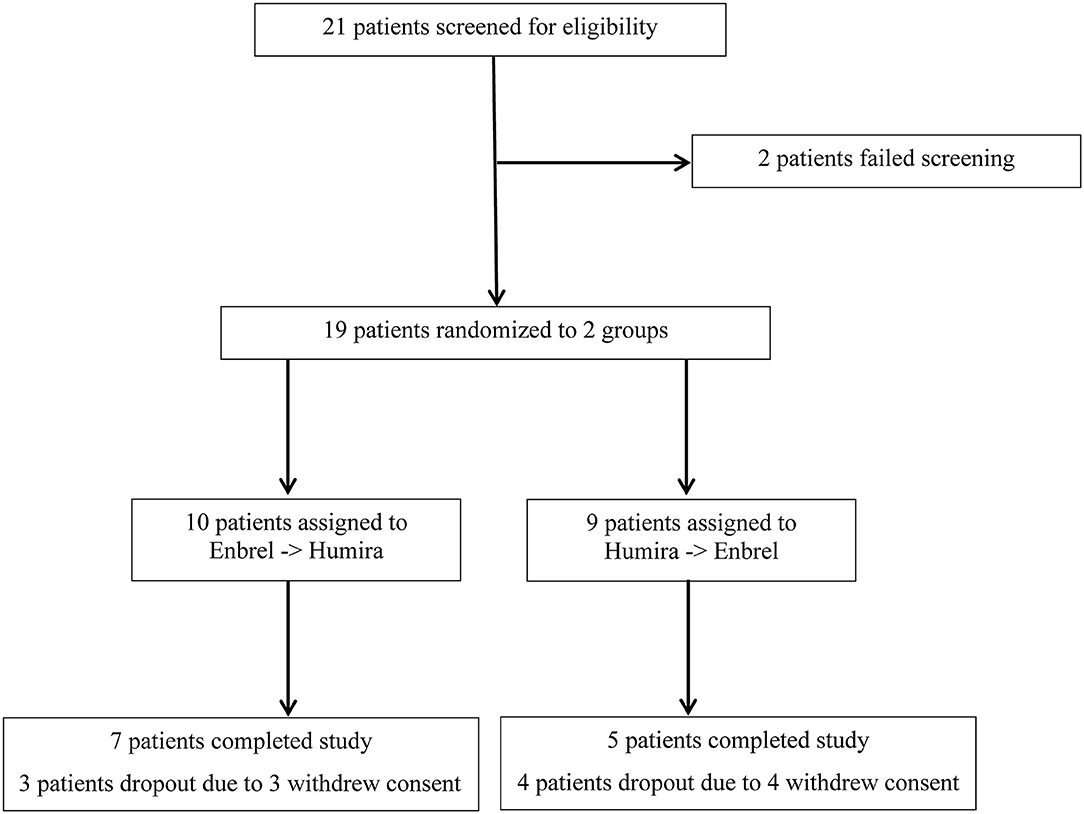
Frontiers Head-to-Head Comparison of Etanercept vs. Adalimumab in the Treatment of Ankylosing Spondylitis: An Open-Label Randomized Controlled Crossover Clinical Trial

PDF) Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration
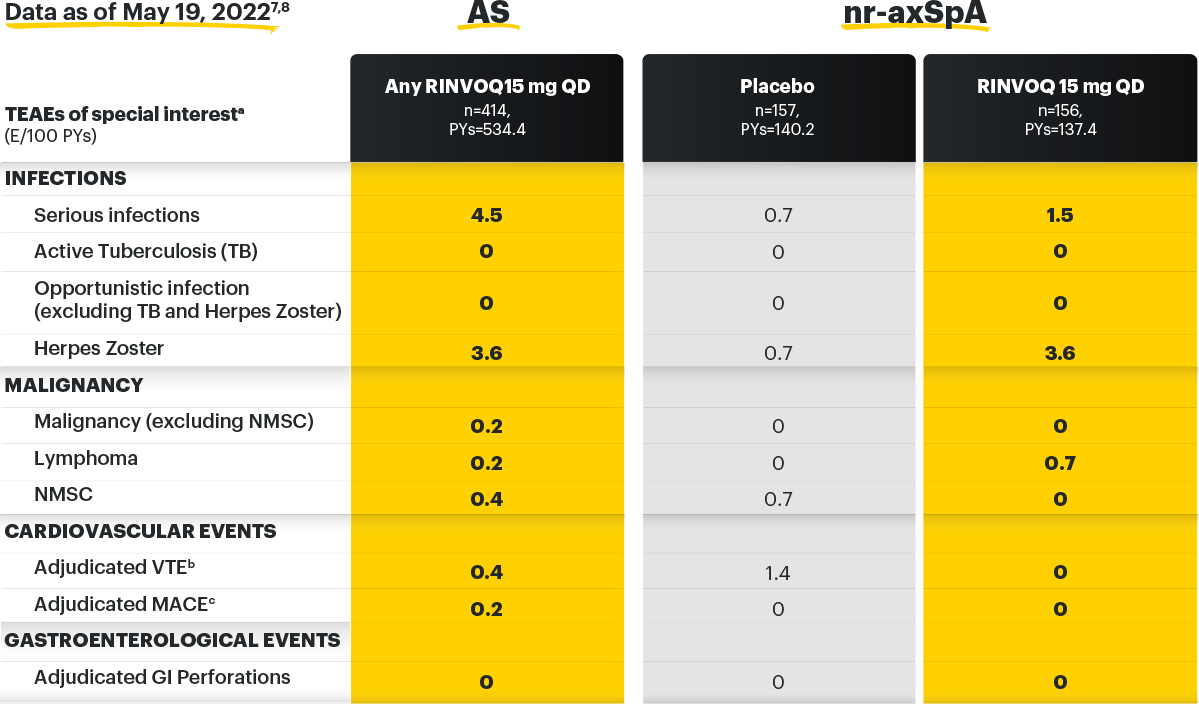
Safety Profile, Axial Spondyloarthritis

Oral Abstracts - 2020 - International Journal of Rheumatic Diseases - Wiley Online Library

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis
Baseline predictors of (A) ASDAS ID and (B) ASAS PR at week 12 of

Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial

Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. - Abstract - Europe PMC

Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. - Abstract - Europe PMC

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis

Treating to Target(s) With Interleukin-17 Inhibitors - Charles W. Lynde, Jennifer Beecker, Jan Dutz, Cathy Flanagan, Lyn C. Guenther, Wayne Gulliver, Kim Papp, Proton Rahman, Dalton Sholter, Gordon E. Searles, 2019
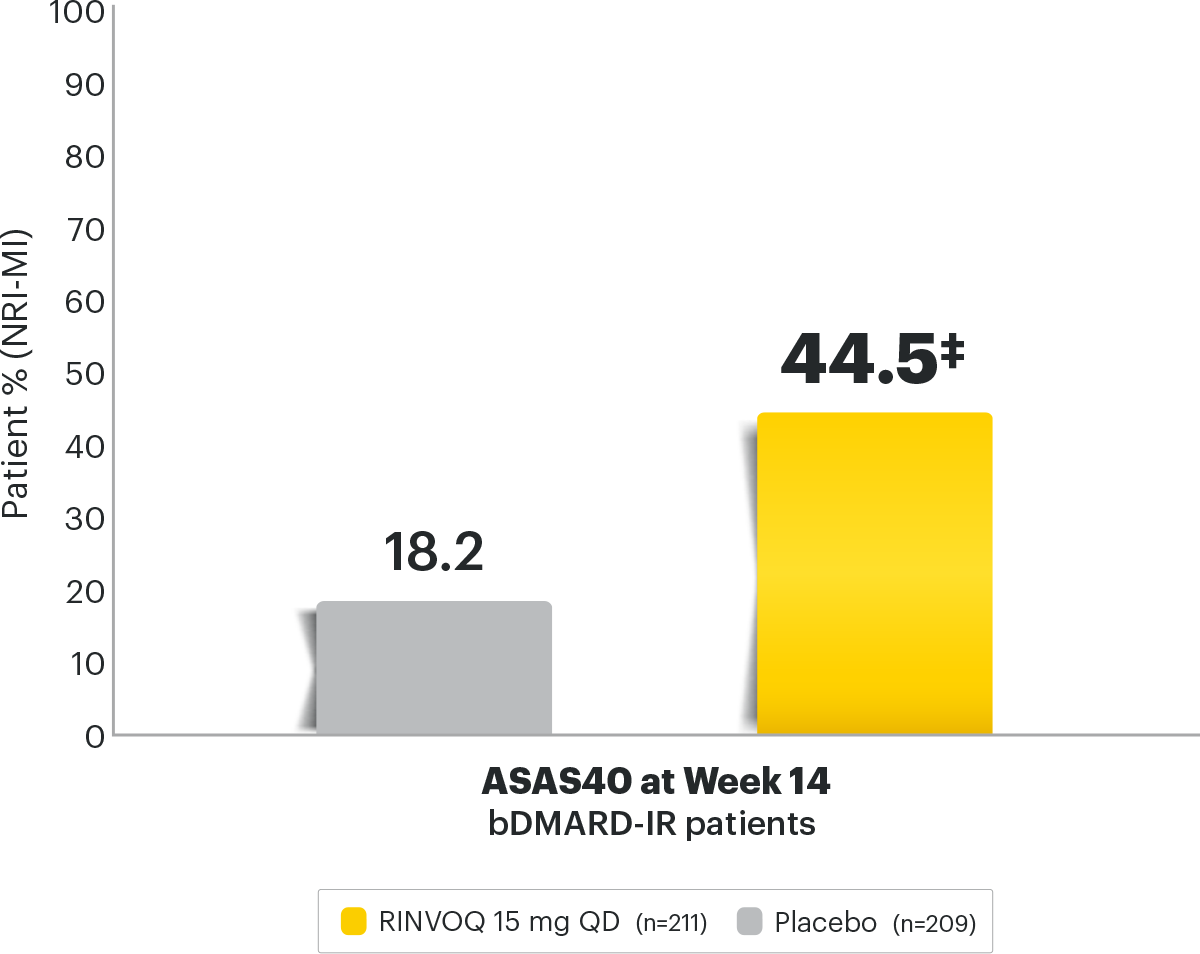
Axial Spondyloarthritis RINVOQ® (upadacitinib)

Long-Term Safety and Efficacy of Ixekizumab in Patients With Axial Spondyloarthritis: 3-year Data From the COAST Program
SIMPONI ARIA® Ankylosing Spondylitis: ASAS Response Rates
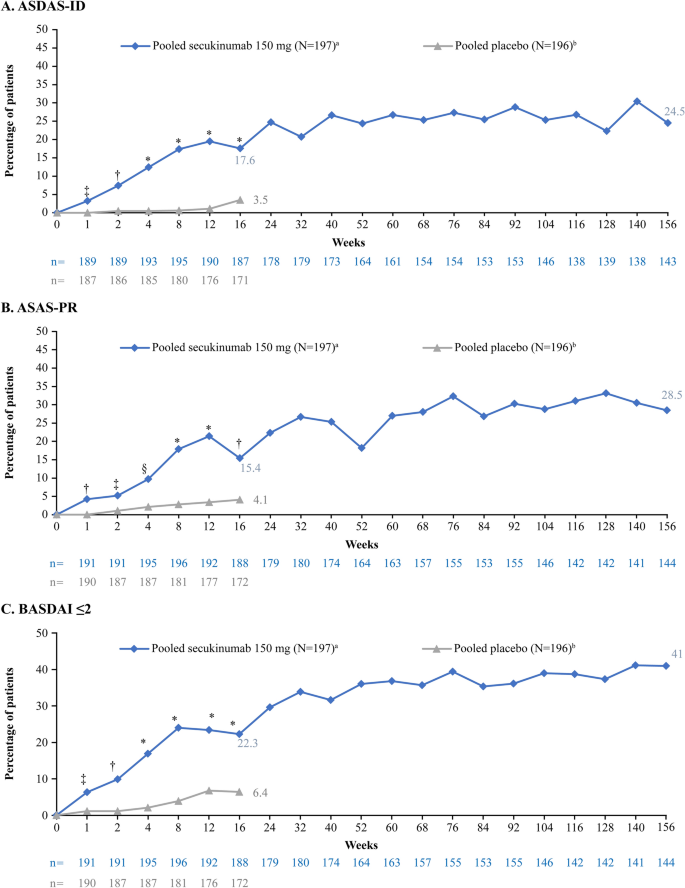
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies
de
por adulto (o preço varia de acordo com o tamanho do grupo)







