Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Annalee Armstrong - Journalist Profile - Intelligent Relations

Annalee Armstrong - Journalist Profile - Intelligent Relations

HBV replication inhibitors - ScienceDirect
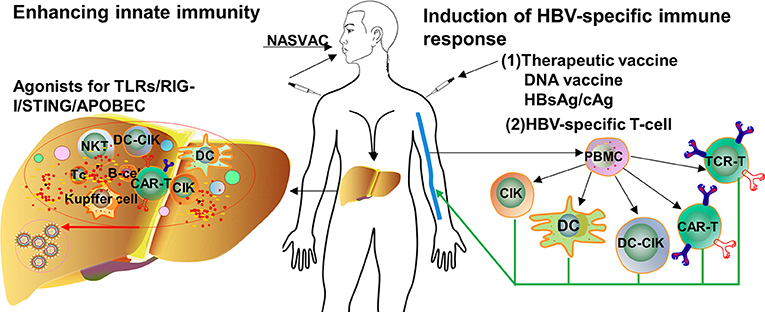
Frontiers Advances in Targeting the Innate and Adaptive Immune

IHEP (International Hepatology Education Program)
33rd Annual Meeting & Pre-Conference Programs of the Society for
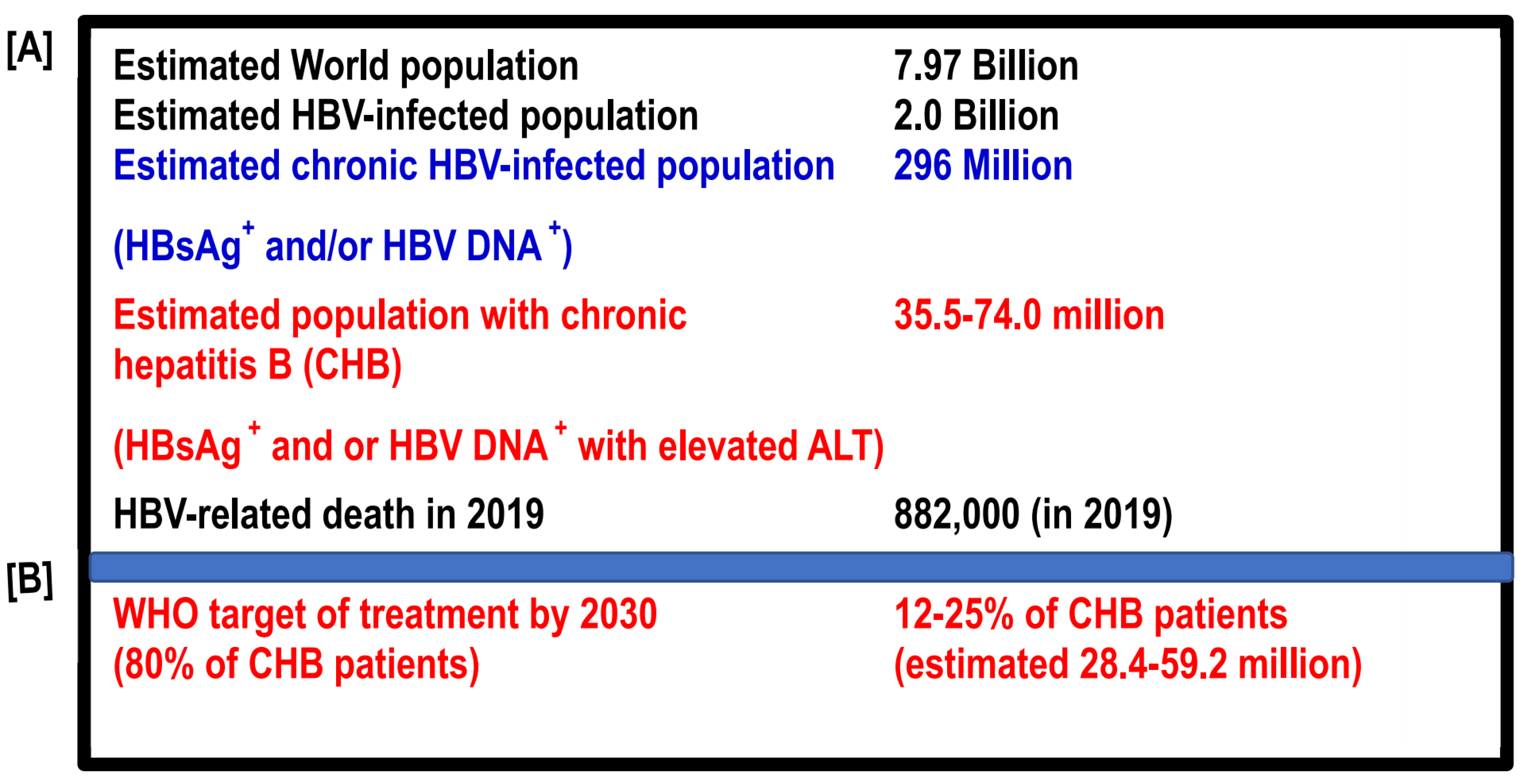
Vaccines, Free Full-Text

HBV replication inhibitors. - Abstract - Europe PMC
Biotechs jockey for gene therapy lead with hemophilia data

Former J&J R&D chief Mathai Mammen lands at FogPharma
de
por adulto (o preço varia de acordo com o tamanho do grupo)





/i.s3.glbimg.com/v1/AUTH_08fbf48bc0524877943fe86e43087e7a/internal_photos/bs/2023/m/t/E533ruToW2TgxpyyyaBA/lancamentos-semana-street-fighter-6-luta-capcom-luke-online-demo.jpg)

