Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Descrição

Design, immunogenicity and efficacy of a Pan-SARS-CoV-2 synthetic DNA vaccine

A first-in-human trial on the safety and immunogenicity of COVID-eVax, a cellular response-skewed DNA vaccine against COVID-19: Molecular Therapy
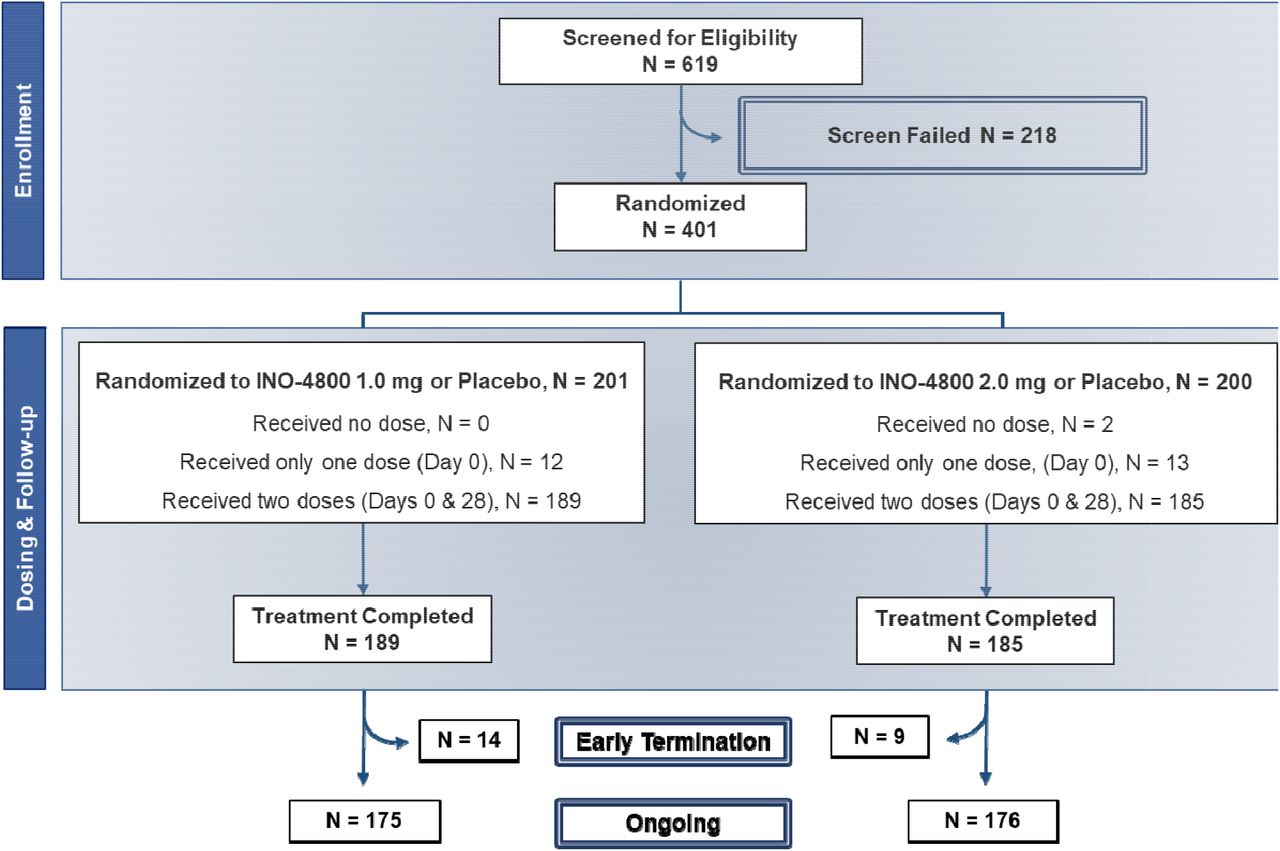
Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure

A brief review on DNA vaccines in the era of COVID-19
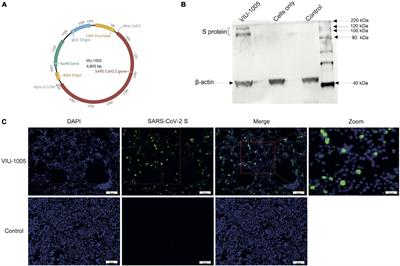
Frontiers Synthetic SARS-CoV-2 Spike-Based DNA Vaccine Elicits Robust and Long-Lasting Th1 Humoral and Cellular Immunity in Mice

Design, immunogenicity and efficacy of a Pan-SARS-CoV-2 synthetic DNA vaccine
China's CanSino pushes coronavirus vaccine into clinical testing as Moderna kicks off trial
DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice

Safety and immunogenicity of two recombinant DNA COVID-19 vaccines containing the coding regions of the spike or spike and nucleocapsid proteins: an interim analysis of two open-label, non-randomised, phase 1 trials in
de
por adulto (o preço varia de acordo com o tamanho do grupo)